

< Previous | Contents | Next >
Section 2 Preliminary Approval
201. General
1. Preliminary approval is a validity approval process on generic design, and the order approval process is specified as Fig 3.3.
of preliminary
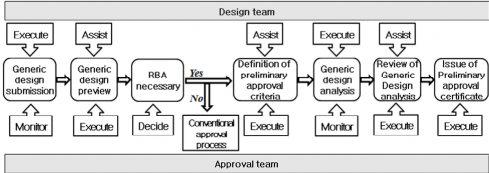
Fig 3.3 Preliminary approval process flow
2. This section may also apply to Approval in Principle(AIP).
202. Submission of generic design
1. The design team is to prepare novel design or risk-based design and submit it and relate docu- ments to the approval team.
2. Overall shape of the ship, configuration of main compartments, structural configuration of main part, material, material and dimension of primary members, arrangement of primary systems, boun- dary condition, main specification, realizing methods of target function and operation characteristics are to be included in generical design.
3. The design team
is to identify existing standards, regulations and rules that the design deviated
from, and explain them to the approval team.
![]()
4. If necessary, terms and meanings concerning design and approval procedure are to be defined at the initial stage. The definition of these terms increases efficiency by avoiding misinterpretations and confusion.
203. Preview of generic design
1. The approval team previews the submitted generic design with respect to safety of life and environ- ment protection and understands clearly the characteristics of the target design. The team considers if risk-based approval procedure is to be applied to the subjected design.
2. The approval team and the design team are to discuss the contents of the submitted generic design at the generic design preview phase. The aim of the generic design preview phase is also to decide whether the risk-based design challenges any existing rules, regulations or standards to such an ex- tent that a risk analysis is required. If risk-based approval procedure is decided not to be applied by an approval team, existing prescriptive regulations and rules may be applied to approve.
3. The
approval team and the design team are to identify and describe items requiring special atten-
tion and to plan how to handle these items with respect to approval.
4. The decision whether the design requires a risk-based approval may be reached by using Table 3.1
to determine the degree of novelty.
![]()
Table 3.1 Categorization of novelty
Technology status Application area | Proven | Limited field history | New or unproven |
Known | 1 | 2 | 3 |
New | 2 | 3 | 4 |
5. A Matrix as shown in Table 3.2. may applied for guidance to the design team when performing preliminary estimates on the extent of work to be performed and submitted for approval.
204. Definition of preliminary approval criteria
1. The approval team is to define the required conditions and formal procedures based on the results on preview discussion of generic design.
2. The specifications which are to be included, but are not limited to, in preliminary approval criteria are as follows:
(1) Definition of main risk concerned, and its evaluation criteria
(2) HAZID plans (including methods and scopes)
(3) Risk analysis and assessment plans (including methods and scopes)
(4) Work plan for generic design analysis such as experiments, calculations, analysis, simulation tests, etc. (including methods and scopes)
(5) Functional requirement and safety requirement list (Draft requirements)
(6) Assumptions, exemptions and restrictions
3. The approval team defines preliminary approval criteria to advise to the design team. Preliminary approval criteria are defined through several meetings led by the approval team and the design team is to participate in the discussions.
4. The following agenda is to be discussed in preliminary approval criteria meeting.
(1) Design concepts
(2) Design objectives
(3) Design novelty
(4) Risk-based features in design
(5) Violations and potential violations related to the existing prescriptive regulations, rules and standards, etc.
(6) The deficiencies or limitations of existing prescriptive regulations, rules and standards
(7) Potential hazards which may happen to all systems and functions of ships
![]()
(8) Necessary items to realize target functions
![]()
Table 3.2 Level of detail of risk-based approval corresponding to degree of novelty
Category | Novelty 1(1) | Novelty 2(2) | Novelty 3(2) | Novelty 4(3) |
Risk Assessment | Not required | Required (partly) * Violation of primary regulations * Depending on risk assessment outcome * Semi-quantitative risk assessment on hazards medium or high(4) | Required (partly) * Quantitative risk assessment on hazards medium or high(5) | Required (overall) * Quantitative risk assessment on all hazards(5) |
Qualifications of a design team | Not required | Required * Design, operational experience experts * Individuals having general knowledge of risk assessment | Required * Design, operational experience experts * Risk assessment experts | Required * Design, operational experience experts * Risk assessment experts |
Applicable regulations | *Existing prescriptive regulations and rules | * Existing prescriptive regulations and rules * Parts of risk-based approval process * Applicable standards if available from other industrial sectors | * Risk-based approval process * Applicable standards if available from other industrial sectors | * Risk-based approval process |
Surveys | *Existing regulations of surveys | * Internal surveys * Additional surveys at related safety events | * Internal/external surveys * Additional periodic surveys at hazards | * Continuous monitoring and reporting |
Note : (1) ‘Novelty 1’ refers to a conventional design and the risk-based approval process need not be applied un- less having a special intention or reason. Therefore existing prescriptive regulations, rules and standards may be applied. (2) ‘Novelty 2-3’ correspond to the middle level not belonging to Novelty 1 and 4 and may take existing approval process and risk-based approval process depending on the situations. Basically, they follow ex- isting approval process according to design and may apply risk-based approval process only for im- portant part systems and functions. If necessary, they may apply risk-based approval process for overall design. (3) ‘Novelty 4’ correspond to a design with a totally new concept which is lack of experience and verification. As there is no existing prescriptive regulations, rules and standards applicable to this, this is to follow risk-based approval process except special cases. (4) Semi-quantitative risk assessment is a method of quantitative estimates. It defines indexes on frequency and consequences of accidents in advance to give an index of the most appropriate grade. (5) Quantitative risk assessment is a method of completely estimating quantitative values. It estimates fre- quency and consequences of accidents in specific numbers through records, experiments, calculations, simulation tests etc. | ||||
5. Appropriate types of risk-based or reliability analysis techniques are to be identified in risk analysis and assessment plans. The features, limitations, application methods etc. are to be definitely mentioned.
6. Appropriate types of experiments, theoretical analysis and calculations, possible simulation tests are to be identified in generic design analysis plans. The features, limitations, application methods etc. are to be definitely mentioned.
7. Generic design analysis plans may be changed by results of generic design analysis and they are to be reflected to specify design analysis plans.
8. Risk of major interest is to be identified, proper evaluation criteria corresponding to the risk are to be defined and identified.
![]()
9. Objective safety level inherent in existing prescriptive regulations, rules and standards etc. may be
![]()
referred to define risk evaluation criteria. Available risk evaluation criteria in other industrial sectors may be referred to. However, an approval or design team is to newly develop specific risk evalua- tion criteria in case of novel design.
205. Analysis of generic design
1. The design team is to analyse risks involved in generic design relating to preliminary approval cri- teria under the supervision of the approval team. The team identifies design items which require specific analysis, carries out experiments, calculations, analysis and simulation tests etc.
2. If necessary, analysis results of generic design may be utilized to risk analysis works again and risk assessment is to be carried out by preliminary approval criteria. Safety of generic design is to be judged.
3. At a minimum, a hazards identification is to be included in risk analysis works. Besides, other risk analysis methods such as FSA, FMECA, HAZOP, FTA, ETA, structural reliability analysis etc. may be applicable.
4. The design team is to carry out hazards identification on novel design or risk-based design. Related hazards and consequences are to be identified, also safety systems(accident prevention and miti- gation measures) are to be found. Through the procedure, the requirements are to be identified to ensure or improve the design safety. Preliminary approval criteria are to be revised. General risk analysis flow is specified as Fig 3.4.
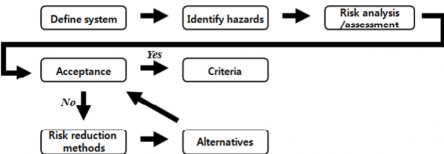
Fig 3.4 Risk analysis flow
5. The design team is to carry out risk assessment at the end, after preparing risk model based on risk analysis results. Risk control measures are to be prepared according to risk assessment results and risk evaluation criteria defined in preliminary approval criteria.
6. The specifications to be considered in risk assessment works. are as follows:
(1) Identified hazards, frequency, consequence
(2) Safety systems included in the design which may be identified
(3) Risk model for quantitative risk analysis
(4) Reference, assumption, uncertainty, sensitivity etc.
(5) Comparison of the calculated risk levels and evaluation criteria
(6) Risk control measures and risk reduction level
(7) Items which need additional risk analysis, experiments, calculations, analysis, simulation tests.
(8) Notices related to construction, operation
7. Documents related to procedures and results are to be properly prepared, because risk assessment results are the most important data for preliminary approval.
206. Review of analysis results for generic design
1. To ensure rationality and adequacy of process and results of generic design, the approval team is to review hazards identification results and confirm that:
(1) participants have appropriate qualifications;
![]()
(2) standard procedures(eg. 「Guidelines for Formal Safety Assessment(FSA) for use in the IMO
![]()
Rule-Making Process(MSC-MEPC.2/Circ.12)」) for hazards identification works were followed.
(3) the rank of identified hazards is considered properly.
(4) the identified safety systems are reflected to the design properly.
(5) features of novel design or risk-based design are reflect to identified hazards and safety systems properly.
(6) if necessary, preliminary approval criteria are revised according to identified design requirements.
2. The approval team is to review rationality and adequacy of risk model
3. The approval team is to review rationality and adequacy of risk assessment results.
4. The approval team is to review rationality and adequacy of risk reduction level where risk control measures are applied by the design team.
5. The approval team is to review that experiments, calculations, analysis, simulation tests etc related to the generic design have been carried out appropriately and transparently, and their results are reasonable.
207. Issuance of preliminary approval certification
1. The approval team is to review the analysis results for generic design in accordance with prelimi- nary approval criteria and decide issuance of preliminary approval certification after judging realiza- tion possibility and safety level of generic design. The certification is not to be issued until all hazards, faults related to generic design are identified and their control is demonstrated.
2. The approval team is to consider the following specifications to issue preliminary approval certification.
(1) Possible to realize generic design
(2) May all identified and calculated risk levels be acceptable
(3) Are there omitted or ignored risks
(4) Comply with design requirements
(5) Is risk reduction level by risk control measures reasonable
3. It is noted that preliminary approval certification is not guaranteed for final approval. It is only possible through the final approval.
4. The form of preliminary approval certificate is to be in accordance with the form defined in
Annex 2.